Benutzer:Mathieu Kappler/Sandkaste/7
| Strukturformel | ||||||||
|---|---|---|---|---|---|---|---|---|
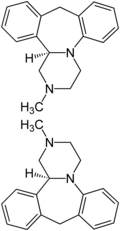
| ||||||||
| 1:1-Gmìsch vum (R)-Isomer (owa) un em (S)-Isomer (unter) | ||||||||
| Allgemeines | ||||||||
| Freiname | Mianserin | |||||||
| Andere Namen |
(RS)-2-Methyl-1,2,3,4,10,14b-hexahydrodibenzo[c,f]pyrazino[1,2-a]azepin | |||||||
| Summeformle | C18H20N2 | |||||||
| CAS-Nummer |
| |||||||
| PubChem | 4184 | |||||||
| ATC-Code | ||||||||
| DrugBank | DB06148 | |||||||
| Arzneistoffaagoobe | ||||||||
| Wirkstoffklass | ||||||||
| Äigeschafte | ||||||||
| Molari Masse | 264,36 g·mol−1 | |||||||
| Aggregatzuestand |
fäscht | |||||||
| Schmelzpunkt | ||||||||
| Sicherhäitshiiwiis | ||||||||
| ||||||||
| Toxikologischi Date | ||||||||
| Sowit wie möglig, wärde SI-Äihäite verwändet. Wenn nüt anders stoot, gälte d Date wo aagee si bi Standardbedingige. | ||||||||
{[{Dialäkt|Elsässisch|Elsassisch|Owerelsassisch}}
D’ Mianserin ìsch a Àrznäischtoff vu dr Grupp vu da tretraziklischa Narvahailmìttel. ’S ìsch a Ànàloogon vu dr Mirtazapin.
Gschìcht
ändereÌm Vergliich mìt da triziklischa Substànza han d’ äältra tretraziklischa kä ärheebliga Fortschrìtt ìn Vertragligkait un Wìrksàmkait. D’ Mirtazapin, a Vàrianta vu dr Mianserin, ìsch àwwer àls Vertratter vun’ra bschtìmmta Grupp, d’ NaSSA. D’ Mianserin, wo sehr ahnlig wia d’ Mirtazapin wìrkt, gheert eewafàlls züa dara Grupp. D’ Mianserin ìsch ànna 1967 vun Organon patentiart worra.[4] Züagloo ìsch sa ànna 1975 ìm Ditschlànd unter’m Màrkanàmma Tolvin, un ìn dr Schwiz un ìm Eeschtriich àls Tolvon. D’rzüa ìsch sa vu zàhlriicha Handler àls Hailmìttel vum äffentliga Gebiat vermarktet. It is also available throughout the world under a variety of other brand names including Athymil, Bonserin, Bolvidon, Deprevon, Lantanon, Lerivon, Miansan, Serelan, Tetramide, and Tolvin among others.[5][6][7]
Mianserin is not approved for use in the United States, but is available in the United Kingdom and other European countries.[8][9] A mianserin generic drug received Vorlage:Abbrlink approval in May 1996 and is available in Australia.[10]
D’ Mianserin ìsch ainer vu da äärschta Narvahailmìttel gsìì, wo-n-uff’m britischa Màrkt ààkumma ìsch, un wo weniger gfoohrlig gsìì ìsch àss d’ triziklika Narvahailmìttel, wänn man ’s ìn Ìwwerdoosa nìmmt. Jedoch ìsch d’ Mianserin numma salta verschrìewa ìm Verainigta Keenigraich.[15]
Wackselwìrkung
ändereMianserin appears to exert its effects via antagonism of histamine and serotonin receptors, and inhibition of norepinephrine reuptake. More specifically, it is an antagonist/inverse agonist at most or all sites of the histamine H1 receptor, serotonin 5-HT1D, 5-HT1F, 5-HT2A, 5-HT2B, 5-HT2C, 5-HT3, 5-HT6, and 5-HT7 receptors, and adrenergic α1- and α2-adrenergic receptors, and additionally a norepinephrine reuptake inhibitor.[41][42] As an H1 receptor inverse agonist with high affinity, mianserin has strong antihistamine effects (e.g., sedation). Conversely, it has low affinity for the muscarinic acetylcholine receptors, and hence lacks anticholinergic properties.[34] Mianserin has been found to be a low affinity but potentially significant partial agonist of the κ-opioid receptor (Ki = 1.7 μM; EC50 = 0.53 μM),[40] similarly to some tricyclic antidepressants (TCAs).[43]
Blockade of the H1 and possibly α1-adrenergic receptors has sedative effects,[44] and also antagonism of the 5-HT2A and α1-adrenergic receptors inhibits activation of intracellular phospholipase C (PLC), which seems to be a common target for several different classes of antidepressants.[45] By antagonizing the somatodendritic and presynaptic α2-adrenergic receptors which function predominantly as inhibitory autoreceptors and heteroreceptors, mianserin disinhibits the release of norepinephrine, dopamine, serotonin, and acetylcholine in various areas of the brain and body.
Along with mirtazapine, although to a lesser extent in comparison, mianserin has sometimes been described as a noradrenergic and specific serotonergic antidepressant (NaSSA).[46] However, the actual evidence in support of this label has been regarded as poor.[47]
Àm Ààfàng wìrkt d’ Mianserin maischt dämpfend. D’ Ìndikàzioona vu dr Mianserin sìnd fascht d’ gliicha wia da vu da triziklischa Narvahailmìttel vu dr Imipramin-Typ. D’ mìttlera Tàgesmänga ìsch vu 30 bis 90 mg. Waga da meeglischa Naawawìkunga soll ma wahrend uff a reegelmassiga-n-Àrt s’ Blutbìld lüaga. D’ Hàlbwartzitt vu dr Mianserin ìsch äbb 17 Schtunda.[48] Wänn dr Pàziant grippa-n-ahnliga Symptooma hàt, Agranulozytose soll ma s’ Medikamant sofort .
Ma hàt schun aimol versüacht, d’ Laaweszitt vu Fààdawìrm dur mehrera Sübschtànza z’ verlängra. Doo drbi ìsch d’ Mianserin wìrklig gsìì.[49]
Naawawìrkunga
ändereUffheerung vu dr Behàndlung dur Mianserin kààt mìt verschìedena Wìrkunga: Narvazammabruch, Àngschtzäschtànd, Panikattacke,[50] nìedra Appetit odd’r sogàr Anorexie, Insomnie, Durchfall, Übelkeit, Erbrechen, so wia grippa-n-ahnliga Symptooma, wia Allergie un Juckreiz, unteràndrem.
A Ìwwerdoosa vu Mianserin kààt Schmarzruckgàng, tiafa Bewusstloosigkait, nìedra Blüatdruck, hoocha Blüatdruck, Harzjààga verursàcha, so wia-n-a Verlängerung vu dr QT-Zitt.[51]
Gegahailààzaiga un Wàrn
ändereàndra Narvahailmìttel ìsch d’ Mianserin fìr d’ Behàndlung vu Narvakrànkhaita nìt ààgazaigt. D’ Mianserin màcht weeniger vegetàtiva Naawawìrkunga wia maischt triziklischa Narvahailmìttel. Trotzdam kààt sa laawendsgfoohrliga Schteerunga verursàcha, wia Blutbìldungsschteerunga, drunter Agranulozytose, odd’r Schaada àm Knochamàrk). Wagadam ìsch d’ Mianserin ìwwerhàuipt nìt àls Ärschtwàhlmìttel fìr d’ Behandlung vu dr Narvazammabruch bnutzt.
Kemii
ändereStereoisomerii
ändereD’ Mianserin sätzt ma-n-àls a Ràzemàt ii, so zu sààga, àls a 1:1-Gmìsch vu dr (S)-Form un vu dr (R)-Form, obwohl d’ (S)-Form fàrmàkoloogisch àktiver ìsch.[53] A Ràzemàtschpàltung mìt Hìlf vu (+)- odd’r (–)-p-Ditoluoylwiisiira hàt ma-n-ànna 1999 bschrìewa.[54]
wia man ’s härschtällt
ändereA Ìwwerblìck vu da Methooda fìr d’ Härschtällung vu dr Mianserin kààt ma ìn’ma Nohschlààgwark fìnda.[55]. Àui bi dr Ärschtààmäldung hàt d’ Firma Organon mehrera Methooda fìr d’ Härschtällung vu dam Medikamant vorgschlààga.[4] Mìt dr Zitt han sìch zwai Schtràtegia fìr dr Uffbàui vu dr Mianserin un wara hìttz’tàgs ààgwandet:
Uffbàui vum 7-Rìng üss Benzylanilin
ändereDr Waag ìwwer d’ 2-Benzylanilin (1) hàt dr Ärfìnder un wìrd àui ìn dr friahjera Literàtüür zitiart.[56] S’ Dibenzoazepin wìrd ìwwer a Imidchlorid (4) üss’m äntschpraachenda Chloracetanilid (3) mìt Phosphoroxychlorid uffbàuia. Dr Piperazinrìng vum tetraziklischa Rìngsyschteem vu dr Mianserin wìrd üss’m 1,2-Diamin (5), wo dur d’ Umsätzung mìt Methylamin äntschtànda ìsch, mìt Hìlf vu Oxalsiiradiethylester (6) un anschließender Reduktion der Piperazindion-Zwischenstufe (7) mit Diboran zum Zielmolekül (8) gschlossa. Dia Syntheesa vu Piperazin ìsch uffwandig àwwer selektiv, ìsch noch hìtt ààgwandt.[57]
Ànders kààt ma dr Piperazinrìng mìt Hìlf vum 1,2-Dibromethan uffbàuia.[58] S’ Lätscht ìsch àwwer schtàrk krabsärragend. D’ 2-Benzylanilin ìsch àui s’ Màteriàl, wo ma-n-àm Ààfàng bnutzt, fìr d’ kirààla Syntheesa vu dr Mianserin, wo-n-ànna 2015 veräffentligt worra ìsch.[59] Schtàtt Methylamin wìrd Phthalimid iigsätzt un s’ Dibenzazepin, wo ma bikummt, wìrd kirààl hydriart
Uffbàui vum 7-Rìng dur d’ Iifiahrung vun’ra Methyylagrupp
ändereDia Meegligkait fìr dr Uffbàui vum 7-Rìng mìt Hìlf vu 2-Aminobenzylalkohol hàt ma ìn dr schpeetera Pàtantaliteràtüür bschrìewa. Schun ànna 1975 hàt d’ Firma Akzo (wo bis 2007 d’ Mutterunternamma vun Organon gsìì ìsch), a Verfàhra ààgmäldet,[60] wo dia Methooda ààgwandt worra ìsch. Dia Methooda brüücht numma viar Etàppa: z’äärscht sätzt ma Styroloxid (1) mìt N-Methylaminoethanol (2) um, dernooh chloriert ma ìn dr Zwìscha-n-etàppa (4). Dernooh Umsetzung mìt 2-Aminobenzylalkohol (5) zur Diphenylpiperazin-Zwischenstufe (6) un Ringschluss mit Polyphosphorsäure zum Mianserin (7) synthetisiert.
D’ Härschtällung vu dr Miansering üss Styroloxid (ohna d’ Schutzgruppa)
D’ Härschtällung vu dr Miansering üss Styroloxid (mìt da Schutzgruppa)
Wittera Forschung
ändereD’ Nutzung vu dr Mianserin, fìr d’ Litt mìt Wohrnammungsschteerung halfa, wo düan mìt Narvapìllala bhàndelt wara, hàt ma-n-ìn klinischa Schtudia ; ìsch unklààr gsìì.[65][66]
z’ lasa
änderehttps://www.thieme-connect.com/products/ejournals/abstract/10.1055/s-2007-1014403
https://europepmc.org/article/med/9179635
https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1600-0447.2001.00148.x
https://www.karger.com/Article/Abstract/67127
https://europepmc.org/article/med/1696292
https://bpspubs.onlinelibrary.wiley.com/doi/abs/10.1111/j.1365-2125.1983.tb05877.x
https://europepmc.org/article/med/8843489
https://link.springer.com/article/10.1007/BF00427964
https://www.sciencedirect.com/science/article/abs/pii/S0147651317308606
https://europepmc.org/article/med/9197943
https://onlinelibrary.wiley.com/doi/full/10.1046/j.1471-4159.1997.69031031.x
https://www.sciencedirect.com/science/article/abs/pii/S016773222032064X
https://www.thieme-connect.com/products/ejournals/abstract/10.1055/s-2007-979298
https://www.sciencedirect.com/science/article/abs/pii/S0014299915001703
https://scholar.google.com/scholar?start=90&q=mianserin&hl=de&as_sdt=0,5
https://www.tandfonline.com/doi/abs/10.1185/03007999009111652
https://link.springer.com/article/10.1007/BF00433747
https://link.springer.com/article/10.1016/j.pharep.2015.06.138
https://www.sciencedirect.com/science/article/abs/pii/S0147651315000585
https://onlinelibrary.wiley.com/doi/full/10.1046/j.1471-4159.2003.02238.x
https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1600-0447.1994.tb01484.x
https://dmd.aspetjournals.org/content/27/10/1200.short
https://setac.onlinelibrary.wiley.com/doi/abs/10.1897/05-495R.1
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1364577/
https://europepmc.org/article/med/9347380
https://www.thieme-connect.com/products/ejournals/abstract/10.1055/s-2007-1019559
https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1600-0447.1988.tb05188.x
https://journals.co.za/doi/abs/10.10520/AJA20785135_14604
https://europepmc.org/article/med/1924659
https://bpspubs.onlinelibrary.wiley.com/doi/abs/10.1111/j.1365-2125.1989.tb05392.x
https://link.springer.com/article/10.1007/BF01959033 SUMMARY
https://www.sciencedirect.com/science/article/abs/pii/S0014489414001428
https://europepmc.org/article/med/7046364
https://link.springer.com/article/10.1007/BF00174375
https://jnnp.bmj.com/content/66/4/490.short
https://onlinelibrary.wiley.com/doi/abs/10.1002/hup.470100406
https://bpspubs.onlinelibrary.wiley.com/doi/abs/10.1111/j.1476-5381.2012.02078.x
https://europepmc.org/article/med/6670581
https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1600-0447.1985.tb08075.x
https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1600-0447.1990.tb06499.x
https://www.sciencedirect.com/science/article/pii/0924977X9500014G
https://journals.sagepub.com/doi/abs/10.1177/0960327110364639
https://link.springer.com/article/10.1007/BF00310523
https://journals.sagepub.com/doi/abs/10.1177/030006057800600306
https://journals.sagepub.com/doi/abs/10.1177/030006057700500411
https://journals.sagepub.com/doi/abs/10.1177/096032719101000514
https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1600-0447.1985.tb08078.x
L. Clemmesen, R. Klysner, L. Lauritzen, D. Loldrup, M. Lunde, E. Schaumburg, S. Waarst, P. Bech: Kombinationsbehandling med imipramin og mianserin: en kontrolleret klinisk undersegelse. In: Nordisk Psykiatrisk Tidsskrift. Band 42, Nr. 6, 1988, S. 529–531, doi:10.3109/08039488809103240 (englisch, tandfonline.com [abgerufen am 29. August 2021]).
https://www.tandfonline.com/doi/abs/10.1185/03007998409109588
https://journals.sagepub.com/doi/abs/10.1177/030006058201000303
https://www.tandfonline.com/doi/abs/10.1185/03007998209109779
https://link.springer.com/chapter/10.1007/978-94-011-6701-7_10
https://bpspubs.onlinelibrary.wiley.com/doi/abs/10.1111/j.1365-2125.1983.tb05881.x
https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1600-0447.1985.tb08079.x
Literàtüür
ändere- M. Peet, H. Behagel: Mianserin: a decade of scientific development. In: British Journal of Clinical Pharmacology. 5 supp. 1, 1978, S. 5S–9S, PMID 623702, PMC 1429213 (freie Volltext) – (englisch).
Weblinks
ändereAinzelnohwiisa
ändere- ↑ The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals. 14. Auflage. Merck & Co., Whitehouse Station NJ 2006, ISBN 978-0-911910-00-1, S. 1064.
- ↑ 2,0 2,1 Vorlage:Linktext-Check in dr Datebank vu Sigma-Aldrich, abgruefen am 10. April 2011 (PDF).
- ↑ 3,0 3,1 Mianserin. In: Römpp Online. Georg Thieme Verlag, abgrüeft am 29. August 2021.
- ↑ 4,0 4,1 S’ Pàtant ìsch doo z’ fìnda: DE 1 695 556 (1967). In: Verfahren zur Herstellung von Piperazinderivaten. Willem Jacob van der Burg, Jaques Delobelle (Organon), abgruefen am 29. August 2021 (änglisch).
- ↑ 5,0 5,1 Index Nominum 2000: International Drug Directory. Taylor & Francis, 2000, ISBN 978-3-88763-075-1, S. 689 u. ff. (englisch, google.com).
- ↑ 6,0 6,1 International brands for mianserin. In: Drugs.com. Abgruefen am 20. August 2017 (änglisch).
- ↑ 7,0 7,1 J. Elks: The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer, 2014, ISBN 978-1-4757-2085-3, S. 822 u. ff. (englisch, google.com).
- ↑ Alan J. Gelenberg, Ellen L. Bassuk, Stephen C. Schoonover: The Practitioner's Guide to Psychoactive Drugs. Springer Science & Business Media, 2013, ISBN 978-1-4757-1137-0, S. 39 u. ff. (englisch, google.com).
- ↑ Donald F. Klein, Lewis P. Rowland: Current Psychotherapeutic Drugs. Routledge, 2013, ISBN 978-1-135-06284-2, S. 57 u. ff. (englisch, google.com).
- ↑ AlphaPharm: Lumin Mianserin hydrochloride product information. (pdf) GuildLink Pty Ltd, abgruefen am 29. August 2021 (änglisch).
- ↑ A historical dictionary of psychiatry. Oxford [u.a.]: Oxford Univ. Press 2005, ISBN 978-0-19-517668-1
- ↑ Stahl's essential psychopharmacology : neuroscientific basis and practical application, 4th, Cambridge: Cambridge University Press 2013, ISBN 9781107025981
- ↑ 29. Affective Disorders. In: Clinical pharmacy and therapeutics, 5th, Edinburgh: Churchill Livingston/Elsevier 2012, ISBN 978-0702042935
- ↑ I.K. Morton, Judith M. Hall: Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media, 1999, ISBN 978-0-7514-0499-9, S. 181 u. ff. (englisch, google.com).
- ↑ J. P. Pratt: Clinical pharmacy and therapeutics. Hrsg.: Roger Walker, Cate Whittlesea. 5. Auflage. Churchill Livingston/Elsevier, Edinburgh 2012, ISBN 978-0-7020-4293-5, 29. Affective Disorders, S. 472 (englisch).
- ↑ PDSP Ki Database. In: Psychoactive Drug Screening Program (PDSP). University of North Carolina at Chapel Hill and the United States National Institute of Mental Health.
- ↑ 17,0 17,1 17,2 : Pharmacological profile of antidepressants and related compounds at human monoamine transporters. In: Eur. J. Pharmacol.. 340, Nr. 2–3, 1997, S. 249–58
- ↑ 18,0 18,1 : Molecular biology of 5-HT receptors. In: Neuropharmacology. 33, Nr. 3–4, 1994, S. 275–317
- ↑ 19,0 19,1 19,2 : Standard binding and functional assays related to medications development division testing for potential cocaine and opiate narcotic treatment medications. In: NIDA Res. Monogr.. 178, 1998, S. 440–66
- ↑ : Characterization of 5-hydroxytryptamine1B receptors in rat spinal cord via [125I]iodocyanopindolol binding and inhibition of [3H]-5-hydroxytryptamine release. In: J. Pharmacol. Exp. Ther.. 260, Nr. 2, 1992, S. 614–26
- ↑ : Identification of 5-hydroxytryptamine1D binding sites in human brain membranes. In: Synapse. 3, Nr. 1, 1989, S. 61–6
- ↑ : Molecular pharmacology of 5-HT1D recognition sites: radioligand binding studies in human, pig and calf brain membranes. In: Naunyn Schmiedebergs Arch. Pharmacol.. 337, Nr. 6, 1988, S. 595–601
- ↑ 23,0 23,1 : Agonist and antagonist actions of yohimbine as compared to fluparoxan at alpha(2)-adrenergic receptors (AR)s, serotonin (5-HT)(1A), 5-HT(1B), 5-HT(1D) and dopamine D(2) and D(3) receptors. Significance for the modulation of frontocortical monoaminergic transmission and depressive states. In: Synapse. 35, Nr. 2, 2000, S. 79–95
- ↑ : Comparison of [125I]iodolysergic acid diethylamide binding in human frontal cortex and platelet tissue. In: J. Neurochem.. 53, Nr. 1, 1989, S. 191–6
- ↑ : RS-127445: a selective, high affinity, orally bioavailable 5-HT2B receptor antagonist. In: Br. J. Pharmacol.. 127, Nr. 5, 1999, S. 1075–82
- ↑ : The pharmacology and distribution of human 5-hydroxytryptamine2B (5-HT2B) receptor gene products: comparison with 5-HT2A and 5-HT2C receptors. In: Br. J. Pharmacol.. 115, Nr. 4, 1995, S. 622–8
- ↑ : Pharmacologic characterization of the human 5-hydroxytryptamine2B receptor: evidence for species differences. In: J. Pharmacol. Exp. Ther.. 276, Nr. 2, 1996, S. 720–7
- ↑ : [3H]-BRL 43694 (Granisetron), a specific ligand for 5-HT3 binding sites in rat brain cortical membranes. In: Biochem. Pharmacol.. 38, Nr. 10, 1989, S. 1693–5
- ↑ : Cloning, characterization, and chromosomal localization of a human 5-HT6 serotonin receptor. In: J. Neurochem.. 66, Nr. 1, 1996, S. 47–56
- ↑ : Differences in the central nervous system distribution and pharmacology of the mouse 5-hydroxytryptamine-6 receptor compared with rat and human receptors investigated by radioligand binding, site-directed mutagenesis, and molecular modeling. In: Mol. Pharmacol.. 64, Nr. 6, 2003, S. 1295–308
- ↑ 31,0 31,1 31,2 31,3 31,4 : Discovery of new tetracyclic tetrahydrofuran derivatives as potential broad-spectrum psychotropic agents. In: J. Med. Chem.. 48, Nr. 6, 2005, S. 1709–12
- ↑ : Cloning, expression and pharmacology of a truncated splice variant of the human 5-HT7 receptor (h5-HT7b). In: Br. J. Pharmacol.. 122, Nr. 1, 1997, S. 126–32
- ↑ : The 5-HT7 receptor: orphan found. In: Trends Pharmacol. Sci.. 18, Nr. 4, 1997, S. 104–7
- ↑ 34,0 34,1 34,2 34,3 34,4 34,5 34,6 : Antagonism by antidepressants of neurotransmitter receptors of normal human brain in vitro. In: J. Pharmacol. Exp. Ther.. 230, Nr. 1, 1984, S. 94–102
- ↑ : Cloning, expression, and pharmacological characterization of a human alpha 2B-adrenergic receptor. In: Mol. Pharmacol.. 38, Nr. 5, 1990, S. 681–8
- ↑ : Cloning of the cDNA and gene for a human D2 dopamine receptor. In: Proc. Natl. Acad. Sci. U.S.A.. 86, Nr. 24, 1989, S. 9762–6
- ↑ : Novel ligands for the human histamine H1 receptor: synthesis, pharmacology, and comparative molecular field analysis studies of 2-dimethylamino-5-(6)-phenyl-1,2,3,4-tetrahydronaphthalenes. In: Bioorg. Med. Chem.. 14, Nr. 19, 2006, S. 6640–58
- ↑ 38,0 38,1 38,2 : Interactions of recombinant human histamine H₁R, H₂R, H₃R, and H₄R receptors with 34 antidepressants and antipsychotics. In: Naunyn Schmiedebergs Arch. Pharmacol.. 385, Nr. 2, 2012, S. 145–70
- ↑ : Discovery of a novel member of the histamine receptor family. In: Mol. Pharmacol.. 59, Nr. 3, 2001, S. 427–33
- ↑ 40,0 40,1 40,2 40,3 The atypical antidepressant mianserin exhibits agonist activity at κ-opioid receptors. In: Br. J. Pharmacol.. 167, Nr. 6, November 2012, S. 1329–41
- ↑ Synaptic Effects of Antidepressants: Relationship to Their Therapeutic and Adverse Effects. In: Schizophrenia and Mood Disorders: The New Drug Therapies in Clinical Practice, S. 67–84, Oxford: Butterworth-Heinemann 2000, ISBN 978-0-7506-4096-1
- ↑ Müller G: Target Family-directed Masterkeys in Chemogenomics. In: Chemogenomics in Drug Discovery: A Medicinal Chemistry Perspective. John Wiley & Sons 8 May 2006, ISBN 978-3-527-60402-9
- ↑ : Direct agonist activity of tricyclic antidepressants at distinct opioid receptor subtypes. In: J. Pharmacol. Exp. Ther.. 332, Nr. 1, 2010, S. 255–65
- ↑ Referänz-Fähler: Uugiltige <ref>-Tag; s isch kei Täxt fir s Ref mit em Name TOLVON aagee wore.
- ↑ Antidepressants reduce phosphoinositide-specific phospholipase C (PI-PLC) activity and the mRNA and protein expression of selective PLC beta 1 isozyme in rat brain. In: Neuropharmacology. 43, Nr. 8, December 2002, S. 1269–79
- ↑ : Meta-analysis of noradrenergic and specific serotonergic antidepressant use in schizophrenia. In: Int. J. Neuropsychopharmacol.. 17, Nr. 2, 2014, S. 343–54
- ↑ : A systematic review of the serotonergic effects of mirtazapine in humans: implications for its dual action status. In: Hum Psychopharmacol. 21, Nr. 2, 2006, S. 117–25
- ↑ Volkhard Kurowski: Intoxikationen. In: Jörg Braun, Roland Preuss (Hrsg.): Klinikleitfaden Intensivmedizin. 9. Auflage. Elsevier, München 2016, ISBN 978-3-437-23763-8, Tetrazyklische Antidepressiva, S. 684.
- ↑ M. Petrascheck et al.: An antidepressant that extends lifespan in adult Caenorhabditis elegans. In: Nature. Band 450, 2007, S. 553–556, doi:10.1038/nature05991, PMID 18033297 (englisch).
- ↑ M. Kuniyoshi, K. Arikawa, C. Miura, K. Inanaga: Panic anxiety after abrupt discontinuation of mianserin. In: Japanese Journal of Psychiatry and Neurology. Band 43, Nr. 2, Juni 1989, S. 155–9, doi:10.1111/j.1440-1819.1989.tb02564.x, PMID 2796025 (englisch).
- ↑ D. Taylor, C. Paton, S. Kapur, D. Taylor: The Maudsley prescribing guidelines in psychiatry. 11. Auflage. John Wiley & Sons, Chichester (West Sussex) 2012 (englisch).
- ↑ Mianserin 30 mg film-coated tablets. In: UK Electronic Medicines Compendium. Januar 2014, abgruefen am 29. August 2021 (änglisch).
- ↑ R. M. Pinder, A. M. L. Van Delft: The Potential Therapeutic Role of the Enantiomers And Metabolites Of Mianserin. In: Br.J.clin.Pharmac. Band 15, 1983, PMC 1427891 (freie Volltext) – (englisch).
- ↑ Pàtant: Jackson Roy William, Subasinghe Kamani Rupika (Monash University, Australia; Polychip Pharmaceuticals Pty. Ltd.): WO 99 16 769. In: Resolution of optically-active compounds. Abgruefen am 29. August 2021 (änglisch).
- ↑ Axel Kleemann, Jürgen Engel, Bernd Kutscher, Dietmar Reichert: Pharmaceutical Substances. 4. Auflage. Thieme-Verlag, Stuttgart 2000, ISBN 978-1-58890-031-9 (englisch).
- ↑ W J Van der Burg, I L Bonta, J Delobelle, C Ramon, B Vargaftig: Novel type of substituted piperazine with high antiserotonin potency. In: Journal of Medicinal Chemistry. Band 13, 1970, S. 35–39, doi:10.1021/jm00295a010 (englisch).
- ↑ Hulinska Hana, Polivka Zdenek, Jilek Jiri et al.: Experimental antiulcer agents: N-substituted 2-(4-methyl-1-piperazinyl)acetamides as pirenzepine models and some related compounds. In: Collection of Czechoslovak Chemical Communications. Band 53, 1988, S. 1820 (englisch, cas.cz).
- ↑ Pàtant: Olivie Jacques (Akzono): US 4 217 452 (1980). In: Synthesis for the preparation of tetracyclic compounds. Abgruefen am 29. August 2021 (änglisch).
- ↑ Piotr Roszkowski, Jan. K. Maurin, Zbigniew Czarnocki: The enantioselective synthesis of (S)-(+)-mianserin and (S)-(+)-epinastine. In: Beilstein Journal of Organic Chemistry. Band 11, 2015, S. 1509 (englisch, beilstein-journals.org).
- ↑ Pàtant: Olivie Jaques (AKZO N V Neth): DE 2 505 239 (1975). In: Verfahren zur Herstellung tetracyclischer Verbindungen. Abgruefen am 29. August 2021 (änglisch).
- ↑ Pàtant: Lypacewicz Maria K, Poslinska-bucewka Halina, Smolinska Jadwiga et al. (Instytut Farmaceutyczny, Warszawa): PL 175 287 (1998). In: Preparation of 1,2,3,4,10,14b-hexahydro-2-methyldibenzo[c,f]pyrazino[1,2-a]azepine.; Chem.Abstr. 130, 296 698 (1999). Abgruefen am 29. August 2021 (änglisch).
- ↑ Pàtant: Kisielowski-Ruppert, Lothar; Mörsdorf, Johann Peter; Grafe, Ingomar; Ahrens, Kurt-Henning (HEUMANN Pharma GmbH & Co): Verfahren zur Herstellung von 1,2,3,4,10,14b-Hexahydro-2-methyl-dibenzo[c,f]- pyrazino[1,2-a]azepin und seiner Salze (DE 4 305 659 (1993)). Abgruefen am 29. August 2021 (änglisch).
- ↑ Pàtant: Zhao, Zhenqiao (Shandong Renhetang Pharmaceutical Co., Ltd., Peop. Rep. China): Process for preparation of mianserin hydrochloride. (CN 101 544 644 (2009)). In: Chem.Abstr. Band 1 216 535, 2009 (englisch, espacenet.com [abgerufen am 29. August 2021]).
- ↑ Pàtant: Haider Akhtar, Bollinger Heinrich, Fischer Alan (S. A. SOCHINAZ, Switz.): Procédé de Preparation d’un composé tetracyclique (CH 678 623 (1990)). Abgruefen am 29. August 2021 (änglisch).
- ↑ V. Terevnikov, G. Joffe, J. H. Stenberg: Randomized Controlled Trials of Add-On Antidepressants in Schizophrenia. In: International Journal of Neuropsychopharmacology. Band 18, Nr. 9, 19. Mai 2015, S. pyv049, doi:10.1093/ijnp/pyv049, PMID 25991654, PMC 4576515 (freie Volltext) – (englisch).
- ↑ J. A. Vernon, E. Grudnikoff, A. J. Seidman, T. W. Frazier, M. S. Vemulapalli, P. Pareek, T. E. Goldberg, J. M. Kane, C. U. Correll: Antidepressants for cognitive impairment in schizophrenia--a systematic review and meta-analysis. In: Schizophrenia Research. Band 159, Nr. 2–3, November 2014, S. 385–94, doi:10.1016/j.schres.2014.08.015, PMID 25240772, PMC 4252251 (freie Volltext) – (englisch).
| Bitte tue de Hiwiis zu Gsundheitsthemene biachte! |
[[[Kategorie:Medikamänt]]